MES for Medical Device Manufacturing
The MES for Medical Device Manufacturing from SYMESTIC delivers fully traceable, audit-ready production processes designed for regulated environments. All process data is captured securely and tamper-proof in the cloud, ensuring that every step meets strict compliance requirements. With automated alerts, continuous monitoring, and end-to-end documentation, manufacturers gain the transparency needed to maintain product safety, pass audits, and scale production reliably.
Start your free trial and see how cloud-native MES technology helps you meet regulatory standards without slowing down operations.
Medical-Device-Specific Challenges
Medical device production demands uncompromising documentation accuracy and full process validation. Every material movement, assembly step, and parameter change must be traceable and linked directly to a timestamp and responsible operator.
Even small deviations can lead to safety risks, so critical parameters require continuous monitoring and instant escalation when limits are exceeded. Manufacturers must maintain ISO 13485 and GMP compliance while keeping production efficient, stable, and fully ready for audits at any time.
SYMESTIC supports this with real-time visibility, standardized workflows, and automated quality control embedded directly into daily operations.
Why SYMESTIC
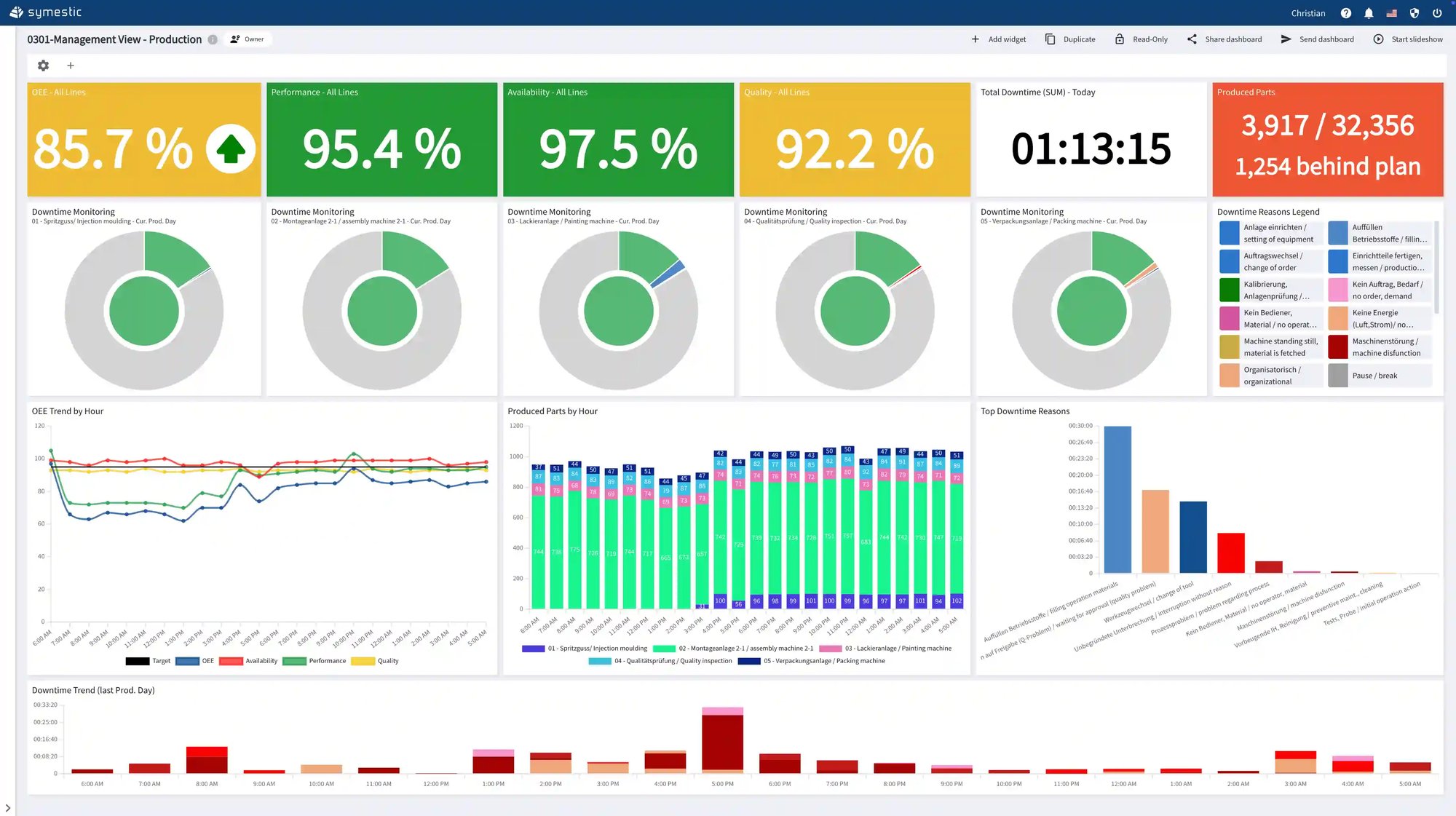
SYMESTIC provides complete digital process documentation across the entire production chain, automatically capturing all relevant parameters and storing them securely with full versioning. This creates a reliable single source of truth for validation, compliance, and audit preparation.
Continuous monitoring ensures that quality-critical conditions remain within specification, while automated alerts enable immediate action should deviations arise. Full traceability connects materials, components, and batches seamlessly, allowing instant impact analysis in case of recalls and ensuring that every production step meets regulatory expectations.
SYMESTIC gives medical device manufacturers the control, transparency, and compliance readiness required for safe, high-quality production.



